Which of the Following Is the Least Acidic Compound
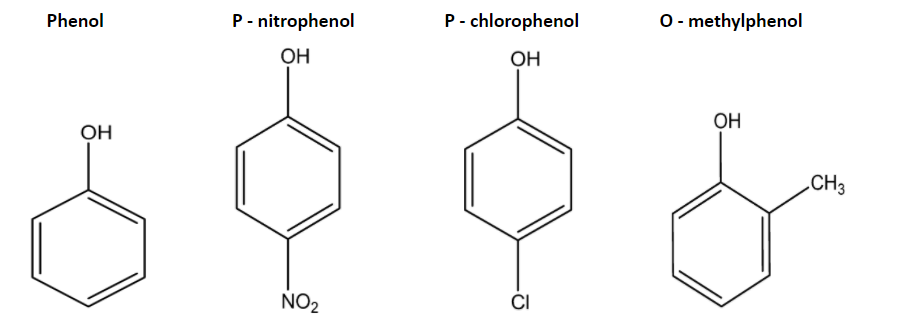
Ethyne is an unsaturated compound consisting of triple bonds due to which both carbon atoms are in. Among these four options p - nitrophenol p - chlorophenol phenol and o - cresol o - cresol is least acidic the order of increasing acidic character among these options are.
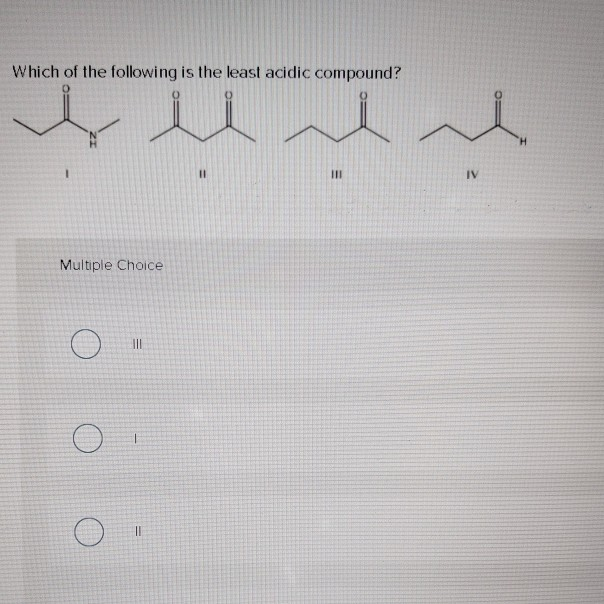
Solved Which Of The Following Is The Least Acidic Compound Chegg Com
First list the ranking number followed by the compound letter in order from 1 to 4 and then explain your reasoning in a sentence or two.
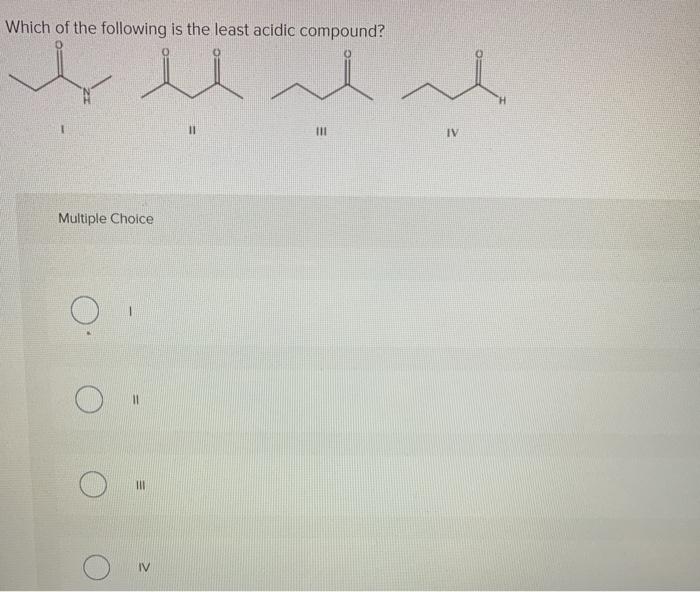
. The release of proton is not easy. In m-nitrophenol the inductive effect of the electron-withdrawing nitro group helps to stabilize the negative charge on oxygen. 3-2-4-1 Rank the following groups of compounds from most acidic 1 to least acidic 4.
Which of the following compounds is least acidic. A Phenol B o-cresol C p-nitrophenol D p-chlorophenol Hard Solution Verified by Toppr Correct option is B o-cresol is least acidic. A Ethanol B p-Nitrophenol C Phenol D m-Nitrophenol.
Asked Dec 24 2021 in Economics by arturoevooo. Which of the indicated hydrogen in the following compounds is the least acidic. A 2-chlorobutanoic acid b 24-dinitrobenzoic acid c acetic acid 4-chlorobutanoic acid.
Which of the following causes the ppf to shift from ppf2 to ppf3. Natalie Preston had the following transactions for Preston Business Services for Year 11 Provided services on account for 300002 Purchased 7500 of supplies on account3 At the end of the year an adjusting entry was prepared for the supplies that had. Among the following which is least acidic.
Rank the following compounds according to acidity 1 being the most acidic and 4 being the least acidic. CH 3 group is electron releasing group. With the least s-character in their orbitals methane and ethane are poor acids in which the carbon atoms are not electronegative enough to stabilize the conjugate base anions.
Depends on what you want to ask least acidic refers to the compound which falls on pH just above 7 ie slightly above neutral alcohols some organic compounds falls in such region cause they can donate H act acidic being physically theoretically neutral. Electron-withdrawing substituents make the phenol more acidic while electron-releasing substituents make the phenol less acidic. Check Answer and Solution for above ques.
-NO 2 exerts a strong -R effect so the acidity gets increased. Chlorine exerts -I and R effect which increases its acidity. A Phenol B o -cresol C p -nitrophenol D p -chlorophenol Hard Solution Verified by Toppr Correct option is B Among the following least acidic is O-Cresol as it has the electron donating group CH 3 at ortho position which destabilises the phenoxide ion and hence less acidic.
Rank the following compounds in order of increasing acidity putting the least acidic first. Leave the remaining answer in each set blank. Rank the following compounds in order of increasing acidity putting the least acidic first.
Rank the following compounds in order of decreasing melting point putting the compound with the highest melting point first. Which of the following species is not a Brønsted-Lowry base. Identify the most and the least acidic compound in each of the following sets.
III I II. Therefore acetic acid is least acidic due to methyl group on carbon of -COOH group. O - cresol is least acidic and it is the correct option from the above information.
A 24-dinitrobenzoic acid is the most acidic compound of this set because it has two strong electronegative substituents nitrogen dioxide which increases the acidity of benzoic acid while p-bromobenzoic acid is the least acidic compound because bromine is a weaker electron withdrawing group than nitrogen dioxide. P-nitrophenol Which of the acids has the weakest conjugate base. P-aminophenol p-cresol p-chlorophenol p-nitrophenol.
3-chlorobutanoic acid p-nitrobenzoic acid p-bromobenzoic acid fluoroacetic acid chloroacetic acid Give IUPAC names for the following. P-methoxyphenol The weakest acid in the table is. From the above table its is clear that p nitro benzoic acid is the most acidic in nature.
More acidic than benzoic acid. Identify the most and the least acidic compound in each of the following sets Leave the remaining answer in each set blank. This is because resonance effect is stronger than the Inductive effect which operates.
A Presence of electron donating group decreases the acidity of carboxylic acid. Asked Aug 24 2019 in Chemistry by Yvonne. III IV II I.
The least acidic compound - p-bromobenzoic acid c The most acidic compound - benzoic acid The least acidic compound - cyclohexanol Explanation. Leave the remaining answer in each set blank. Identify the most and the least acidic compound in each of the following sets.
_ propanoic acid__ b 3-fluoropropanoic acid. - c difluoroacetic acid. It intensifies negative charge on O hence destabilize the phenoxide ion.
Leave the remaining answer in each set blank. Identify the most and the least acidic compound in each of the following sets. Phenols - Physical and Chemical Properties.
Oxalic acid is the most acidic compound due to. Hence option B is correct.

Which Of The Following Is The Least Acidic Compound Brainly In
Which Of The Following Is Least Acidic A P Nitrophenol Class 12 Chemistry Cbse

Solved Which Of The Following Is The Least Acidic Compound Chegg Com
No comments for "Which of the Following Is the Least Acidic Compound"
Post a Comment